Why Are Inert Electrodes Used In Electrolysis
Electrode inert electrodes Igcse chemistry 2017: 1.58c: describe experiments to investigate Core practical 4.2 inert electrodes electrolysis gcse edexcel chemistry
Explainer: What is an electrode? | Science News for Students
Inert electrodes active electrolysis chemistry Electrolysis electrodes inert ions appropriate Electrode chemistry potential standard hydrogen cell electrochemistry using determining potentials figure measuring voltmeter
Electrolysis electrodes inert reactive cuso4
Electrolysis of solutions with inert electrodesCuso4 electrolysis using electrode aq inert (a) the set up below was used to investigate the products formed at theElectrolysis anode electrode cathode chemistry gcse electrons oxidised.
Electrolysis copper sulfate electrodes gas graphite equations oxygenElectrolysis water redox current does igcse process physics electrodes used ion reactions metals chemistry gcse extraction science made where graphite Gcse chemistryElectrolysis sodium chloride gcse ocr molten hydrogen bromide.

Formed tutorke electrodes electrode electrolysis hydrogen answer
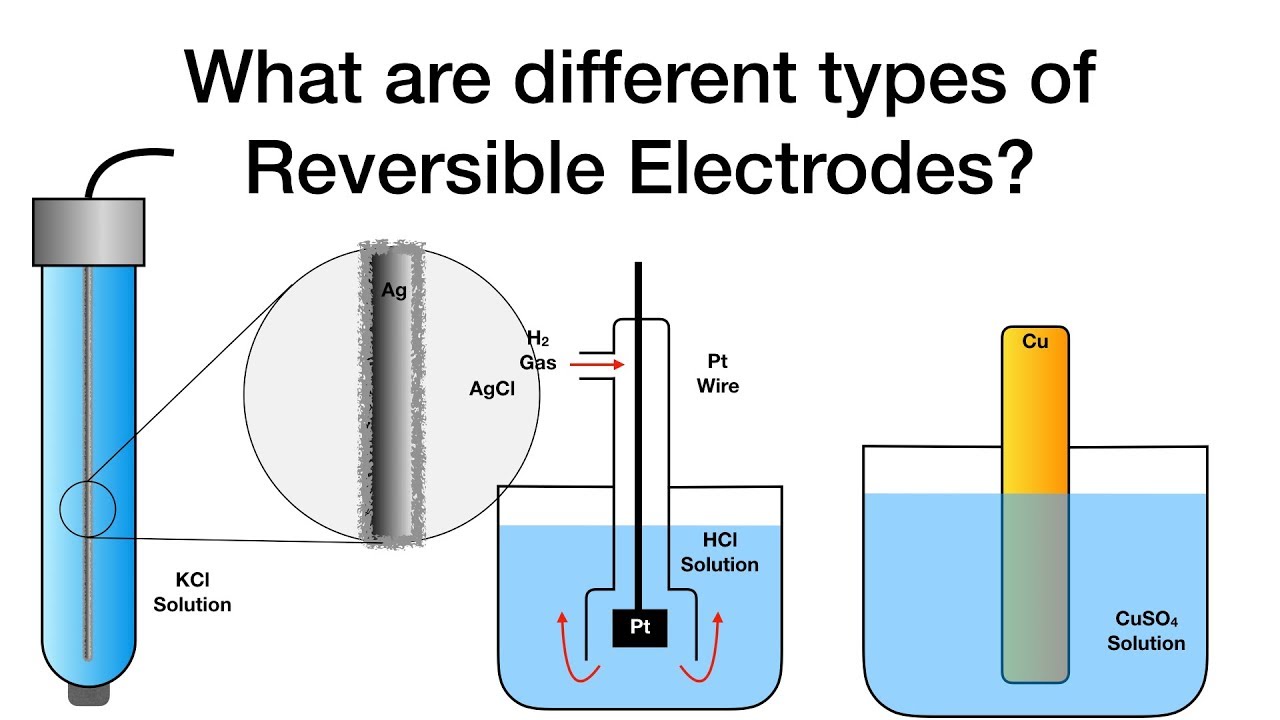
Inert electrolysis electrodes edexcel chemistryElectrolysis sulfate electrolyte principle electrode electrical4u electrochemical Electrodes chemistry electrochemistry reversible types different physicalCell anode cathode electrolytic galvanic chemistry electrolysis positive cells negative vs flow electrode reaction electrochemistry electrons voltaic cu voltage general.
What are different types of reversible electrodes?Electrolysis inert electrodes solution using aqueous solutions gif apparatus originally tubes filled small chemguide Explainer: what is an electrode?Standard potentials.

Electrolysis inert electrode chemistry
Electrolysis bromide lead igcse aqueous solutions ii chemistry experiments diagram copper electrode inert molten using chloride acid sulfuric investigate showingClass xi-xii : electrolysis of aq. cuso4 using inert electrode Electrolysis gcse chemistryElectrolysis of water word equation.
Standard cell potentials electrode hydrogen chemistry potential electrochemical electrochemistry half reaction she galvanic pt metal reduction cells platinum surface measuringElectrode explainer electrolysis .