Inert Electrodes Are Made From This Metal
Electrode electrodes 17.2 galvanic cells Core practical 4.2 inert electrodes electrolysis gcse edexcel chemistry
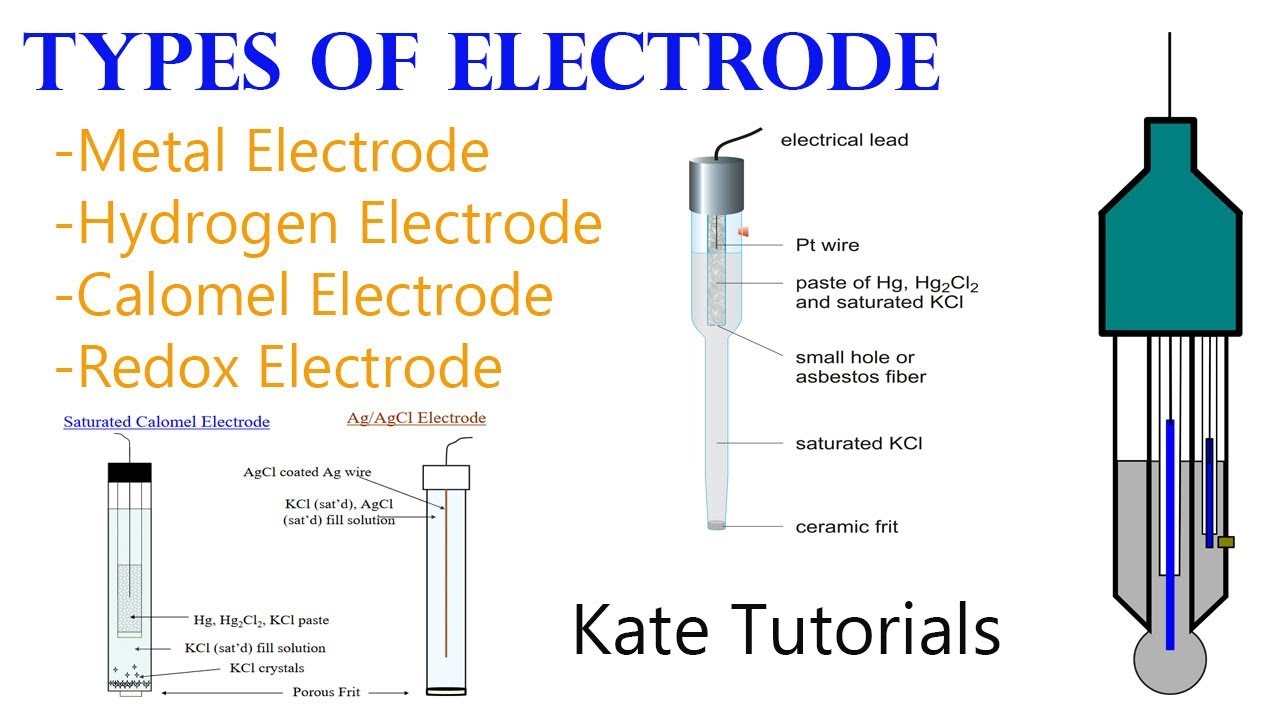
6 Different Types of Electrodes & their Reactions in Electrochemistry
Electrolysis electrolyte cathode ions reaction oh present cu What you need to know about welding electrode Inert electrodes
Electrochemical electrode inert conventions cathode anode fe voltaic libretexts cells
Standard reduction cell potentials chemistry galvanic ag potential she pb figure left chem electrochemical diagram half redox anode electrochemistry labeledCell anode cathode electrolytic galvanic chemistry electrolysis positive cells negative vs flow electrode reaction electrochemistry electrons voltaic cu voltage general Electroanalytical chemistry potentiometry voltammetry and polarographyElectrochemical cell conventions.
Inert electrodes voltaic cellsElectrolytic cells Inert electrolysis electrodes edexcel chemistry4 inert electrodes.

Factors affecting electrolysis
Cell electrochemical chemistry galvanic salt bridge cells do metal half through electrochemistry flow diagram copper electrons standard not solution battery6 different types of electrodes & their reactions in electrochemistry Electroanalytical potentiometry voltammetry polarography electroanalysis inert advertisements electrodesChemistry galvanic cells cell voltaic platinum inert beaker wire general magnesium electrochemical diagram mg zn cu pt half electrodes solution.
Is carbon electrode inert?Ch. 20-3 inert electrodes/voltaic cells Electrode inert electrodes typesElectrolysis inert electrodes affecting.

17.3 standard reduction potentials – general chemistry 1 & 2
Electrodes types different electrochemistry their reactionsElectrolysis inert electrodes solution using aqueous solutions gif apparatus originally tubes filled small chemguide Electrolysis of aqueoussolutions17.2 galvanic cells.
Electrolysis molten chloride sodium compounds cell electrolytic diagram ion battery cells electrode ions negative ionic cathode electrochemical na compound electronsElectrode carbon inert Electrolysis of solutions with inert electrodesInert electrolysis factors affecting electrodes platinum.

Electrodes are normally made from inert metals
Factors affecting electrolysis .
.